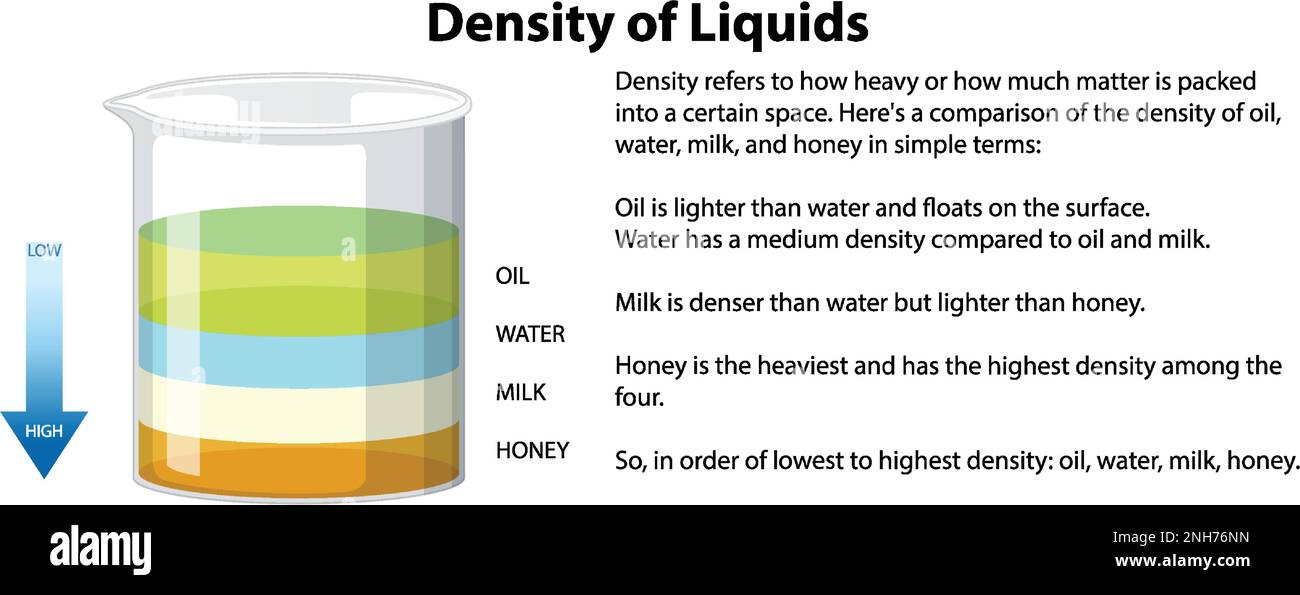
Grasping the Concept of Density: A Simple Explanation
Density is a fundamental property of matter that describes how much mass is packed into a given volume. It’s a measure of how tightly matter is crammed together. Simply put, density is mass divided by volume (Density = Mass/Volume). Understanding how is density used in the real world begins with grasping this basic definition. It is a crucial concept that influences many aspects of our daily lives, from the objects we use to the natural phenomena we observe.
Many people confuse density with weight or heaviness. However, density is not the same as weight. Weight is the force of gravity acting on an object’s mass. Density, on the other hand, is an intrinsic property of a substance. Consider a feather and a rock. A rock is much heavier than a feather. However, this doesn’t necessarily mean the rock is denser. If we had a very large feather and a very small rock, the rock would still be denser. This is because the rock’s mass is packed into a much smaller volume than the feather’s mass. The feather contains a lot of air within its structure, increasing its volume without significantly increasing its mass. The concept of how is density used in the real world becomes clearer when we differentiate it from simple heaviness.
Density is typically expressed in units of grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³). These units reflect the relationship between mass and volume. A substance with a high density has a large mass packed into a small volume, while a substance with a low density has a small mass spread out over a larger volume. The properties of materials dictate how is density used in the real world. Understanding how is density used in the real world requires recognizing its importance in various fields. From engineering to cooking, density plays a crucial role in determining how things behave and interact.
Density in Action: How is Buoyancy Determined?
Density plays a crucial role in determining buoyancy, which is the ability of an object to float in a fluid. An object will float if its average density is less than the density of the fluid it’s placed in. Conversely, an object will sink if its average density is greater than the fluid’s density. This principle explains why a wooden block floats in water, while a metal block sinks. The wooden block has a lower density than water, while the metal block has a higher density. How is density used in the real world? Understanding density helps explain many everyday phenomena.
Consider the example of a ship. Ships are made of steel, a material significantly denser than water. However, ships float because they are designed to displace a volume of water whose weight is greater than the ship’s total weight. The ship’s large, hollow structure increases its overall volume without significantly increasing its mass. This results in a lower average density for the entire ship-water system, allowing it to float. This showcases how manipulating an object’s shape and volume can be used to alter its average density and thus influence its buoyancy. How is density used in the real world to design effective and safe ships? Engineers carefully calculate the volume and mass to ensure the ship floats reliably and safely.
This concept of buoyancy is not limited to water. Objects can float or sink in any fluid, including air. Hot air balloons, for example, float because the heated air inside the balloon is less dense than the surrounding cooler air. This difference in density creates an upward buoyant force, lifting the balloon. Understanding density is therefore essential in various fields, from naval architecture to meteorology. How is density used in the real world to control the flight of hot air balloons? The density difference between the hot air inside and the cold air outside directly determines the balloon’s lift capacity.
Density’s Role in the Kitchen: Mastering Culinary Techniques
Density plays a crucial role in many culinary processes. Understanding how density affects ingredients helps create visually appealing and delicious dishes. How is density used in the real world? A prime example is the layering of cocktails. Liquids with different densities will naturally separate when layered carefully. Heavier liquids, like grenadine or simple syrup, sink to the bottom, while lighter liquids, such as sodas or juices, remain on top. This creates a visually striking and flavorful drink. This principle allows bartenders to create visually stunning layered cocktails, demonstrating how density impacts the presentation and texture of food and beverages. The separation isn’t just for aesthetics; it often enhances the taste experience by allowing for a gradual transition of flavors as the drink is consumed.
Salad dressings offer another excellent illustration of density’s influence. Oil and vinegar, the primary components of many salad dressings, have significantly different densities. Oil, being less dense, floats on top of the vinegar. Shaking the dressing temporarily mixes the components, but given time, the components will separate again. Emulsifiers are used to combat this, creating a homogenous mixture. However, understanding the density differences helps chefs select the right emulsifier for the desired consistency. How is density used in the real world? Many cooking methods, such as creating a perfect soufflé or a flaky croissant, benefit from controlling density. The balance of ingredients, their respective densities, and how these densities change with temperature are crucial to obtaining optimal outcomes. These examples showcase how a basic physical property, density, is fundamental to understanding and improving culinary techniques.
Density also influences the texture and consistency of baked goods. The distribution of ingredients in a cake batter, for instance, relies on the density differences between the various components – flour, sugar, eggs, and fat. The density of these ingredients affects the rising of the cake. In creating a cake, it is crucial to incorporate air pockets which have a lower density than the liquid ingredients, resulting in a lighter and fluffier texture. These factors, which are all dependent on density, determine the final texture and structure of the baked item. A good baker understands how density governs the final result, using this knowledge to refine recipes and deliver consistently satisfying outcomes. How is density used in the real world? In short, it forms the backbone of many culinary processes, affecting both presentation and flavor profile. Mastering these effects elevates culinary skills.
How to Measure Density: A Simple Home Experiment
Understanding how is density used in the real world begins with practical application. Measuring density at home is a simple experiment that demonstrates its core principles. This activity requires readily available tools: a scale (preferably digital for accuracy), a measuring cup or graduated cylinder, water, and the object whose density you wish to determine. Safety is paramount; adult supervision is recommended, especially with young children.
The first step involves measuring the mass of the object. Place the object on the scale and record its mass in grams (g). Next, determine the volume of the object. If the object is regularly shaped (like a cube or a rectangular prism), you can measure its dimensions (length, width, and height) with a ruler and calculate the volume using the appropriate formula. However, for irregularly shaped objects, the water displacement method is more suitable. Fill the measuring cup or graduated cylinder with a known volume of water. Record this initial volume. Gently submerge the object completely in the water, ensuring that no air bubbles are trapped. Record the new water level. The difference between the final and initial water levels represents the volume of the object in milliliters (mL). Since 1 mL is equivalent to 1 cubic centimeter (cm³), you now have the volume in cm³.
With both the mass and volume determined, calculating the density is straightforward. Density is defined as mass divided by volume (Density = Mass / Volume). Divide the mass of the object (in grams) by its volume (in cm³). The resulting value will be the density of the object in grams per cubic centimeter (g/cm³). For example, if an object has a mass of 50g and a volume of 25 cm³, its density would be 2 g/cm³. Knowing how is density used in the real world allows for comparisons. Comparing your calculated density to known densities of common materials can provide insight into the object’s composition. This simple experiment illustrates how density is a fundamental property that links mass and volume, offering a tangible understanding of this important concept. Factors impacting accuracy, such as scale precision and accurate water level readings, should always be considered to ensure reliable results. How is density used in the real world? It’s used for material identification.
Density and Material Identification: A Practical Guide
Density serves as a valuable tool for identifying different materials. The principle behind this application lies in the fact that each substance possesses a unique density at a specific temperature and pressure. This characteristic “fingerprint” allows for comparison and identification when dealing with unknown samples. How is density used in the real world? It’s a key factor in material science.
The process involves determining the density of the unknown substance through experimental measurements. This can be achieved by carefully measuring its mass and volume. Once the density is calculated, it can be compared to a table of known densities for various materials. A close match suggests a possible identification. However, it is important to note that some materials may have similar densities, so additional tests may be necessary for confirmation. How is density used in the real world to identify materials? Comparing measured values with reference tables helps narrow down possibilities.
This technique finds extensive use in various fields. In gemology, density is a crucial parameter for identifying gemstones. For example, a diamond has a significantly higher density than cubic zirconia, a common diamond simulant. Mineralogy also relies heavily on density for identifying minerals. Similarly, industries involved in quality control and material verification employ density measurements to ensure the purity and authenticity of raw materials and finished products. How is density used in the real world to ensure product quality? Density checks offer a reliable method for verifying composition. Furthermore, archaeologists can use density to analyze artifacts and gain insights into their composition and origin. How is density used in the real world across different science fields? From identifying minerals to verifying materials, density offers valuable information. This simple yet powerful concept plays a significant role in numerous scientific and industrial applications. The accuracy of density measurements can be affected by factors such as temperature and impurities, so it is crucial to control these variables carefully to obtain reliable results.
Density in Engineering: Designing for Strength and Efficiency
Engineers consider density a critical parameter in numerous design applications. Understanding how is density used in the real world is essential for constructing safe and efficient structures, vehicles, and systems. The selection of materials hinges significantly on their density, influencing both the structural integrity and overall performance of the final product. Balancing strength and weight is a perpetual challenge, and density plays a pivotal role in achieving this equilibrium.
In civil engineering, density is a key factor in selecting materials for buildings and bridges. High-density materials, such as steel and concrete, offer exceptional compressive strength, making them suitable for supporting heavy loads. However, their weight can also add to the overall load on the structure, potentially requiring more robust foundations and support systems. Therefore, engineers carefully evaluate the density of these materials to optimize structural design and ensure stability. Lighter materials, like aluminum, might be used in specific areas to reduce weight without compromising strength, demonstrating how is density used in the real world to create effective designs. The cost and ease of use of materials must also be considered. How is density used in the real world to guide smart engineering choices?
In aerospace and automotive engineering, minimizing weight is paramount to improving fuel efficiency and performance. Engineers explore materials with high strength-to-weight ratios, such as aluminum alloys, titanium, and composite materials. These materials offer considerable strength while maintaining relatively low densities. This is an important example of how is density used in the real world. Aircraft design benefits greatly from lightweight components, as reduced weight translates into lower fuel consumption and increased payload capacity. Similarly, in the automotive industry, lighter vehicles achieve better fuel economy and improved handling. Material selection considering density is an ongoing process, driven by the need for more sustainable and efficient designs. These design innovations highlight how is density used in the real world to advance technology and sustainability.
Density’s Impact on Weather Patterns: Understanding Atmospheric Processes
Density plays a crucial role in shaping weather patterns and atmospheric processes. Air density variations, driven by temperature and humidity differences, influence air movement and ultimately, weather events. Warm air, being less dense than cold air, rises, creating areas of low pressure. This phenomenon, how is density used in the real world, is the foundation for convection currents.
Convection is a primary driver of weather. As warm air ascends, it cools and can no longer hold as much moisture. This leads to cloud formation and precipitation. Conversely, cold, dense air sinks, creating high-pressure zones. These high-pressure areas are generally associated with clear skies and stable weather. The movement of air from high to low-pressure areas generates wind. Therefore, density differences are fundamental to understanding wind patterns and storm development. How is density used in the real world to forecast weather? Meteorologists use density data to model atmospheric behavior and predict future weather conditions. For example, the interaction of warm, moist air with cooler, drier air can lead to the formation of thunderstorms or even more severe weather events like tornadoes and hurricanes. The density contrasts intensify these systems.
Ocean currents are also influenced by density. Differences in water temperature and salinity affect density, driving the movement of ocean water on a global scale. Cold, salty water is denser and tends to sink, while warmer, less salty water rises. This creates a complex system of currents that distribute heat around the planet. How is density used in the real world to maintain global temperatures? The thermohaline circulation, a major ocean current system driven by density differences, plays a vital role in regulating Earth’s climate. Changes in ocean density, caused by factors like melting ice caps or altered precipitation patterns, can disrupt these currents and have significant consequences for regional and global weather patterns. Therefore, understanding density is crucial for comprehending and predicting long-term climate trends.
Density and Medical Diagnostics: Applications in Healthcare
Medical imaging relies heavily on the principles of density to visualize the human body’s internal structures. Techniques like X-rays and Computed Tomography (CT) scans exploit the varying densities of different tissues to create detailed images. How is density used in the real world within these applications? X-rays, for instance, pass through the body, and the amount of radiation absorbed depends on the density of the tissue. Denser materials, such as bone, absorb more radiation and appear lighter on the X-ray image, while less dense tissues, like lungs filled with air, allow more radiation to pass through and appear darker. This contrast allows medical professionals to identify fractures, tumors, and other abnormalities.
CT scans provide a more detailed cross-sectional view of the body. These scans use X-rays from multiple angles to create a three-dimensional image. The data collected reflects the density of the tissues at each point. Advanced computer algorithms then reconstruct these density variations into a comprehensive image. The ability to differentiate tissues based on their density is crucial for diagnosing a wide range of conditions, from identifying subtle changes in organ structure to detecting the presence of blood clots. How is density used in the real world in CT scans? The precise measurement of tissue density allows for accurate diagnosis and treatment planning.
Bone density scans, also known as DEXA scans, are another significant application of density measurement in healthcare. These scans measure the mineral density of bones to assess the risk of osteoporosis, a condition characterized by weakened bones that are prone to fractures. By quantifying bone density, healthcare providers can identify individuals at risk and recommend appropriate interventions, such as lifestyle changes or medication, to prevent fractures and maintain bone health. How is density used in the real world to improve patient outcomes? Monitoring bone density over time allows for the evaluation of treatment effectiveness and adjustments to management strategies. These scans are a critical tool in preventative medicine, ensuring that individuals receive timely care to maintain their skeletal health and overall well-being. The accurate assessment of density plays a vital role in various diagnostic and therapeutic aspects of modern healthcare.